With a semi-automated double system all sequential steps during the dissolution run are reproducibly executed without requiring user interaction until the post-run activity. Compared to a single system, a double dissolution system will give the user the possibility to increase his throughput by using two dissolution baths, i.e. test 12 samples.
Choose Your Analytical Configuration
Off-line
The dissolution bath can be flexibly combined with additional modules for automated sample collection and storage.
Scalable collection and storage of samples into standardized tubes or vials
Sample protection from temperature and light degradation
Sample dilution
Use of vertical space to reduce footprint
UV-Vis On-line
The dissolution bath can be flexibly combined with additional modules including UV-Vis integration.
Automated UV-Vis measurements for real time results
Single dissolution software for raw data acquisition and flexible calculation
Automates the manual sample transfer which can be a source of manipulation error
Allows unattended dissolution run, results being stored and saved automatically
UV-Vis On/Off-line
The dissolution bath can be flexibly combined with additional modules for automated sample collection, storage, and UV-Vis integration.
Flexibility: fraction collection and/or UV-Vis measurements for sample archival or UV-Vis/LC immediate comparison
AT Semi-Automated UV-Vis On/Off-line (double system)
The new state-of-the-art standard: Xtend™ Dissolution Line – modular, scalable, future-proof
The most flexible and space-saving system to get results on the fly and/or collect samples in LC vials or glass tubes from 2 apparatuses
Driven by 21 CFR, Part 11 compliant ready WinSOTAXplus Advanced Dissolution Software
Use of any major brands of UV-Vis spectrophotometers including the SOTAX Specord Plus models
Options for media replacement, filter and media change, and capacity extension
100% visibility and CenterView™ video monitoring
The innovative circular design of the dissolution bath opens up a new dimension of observation possibilities for R&D visualization and OOS troubleshooting. Understanding the release characteristics of formulations requires full visibility of samples during the dissolution test. Depending on your observation needs, the circular AT bath allows visual observation of each vessel from the outside and additionally from the inside using video monitoring. To view the behavior of samples from the bottom, an optional mirrored base plate is available.
The unique CenterView™ design provides perfect visualization and video recording of release processes in each vessel. Located in the center of the dissolution bath, all cameras can be adjusted and controlled for height and focal distance. For optimum visibility, indirect light is integrated into this space-saving design.
Precise temperature control and easy handling & cleaning
The innovative circular design of the dissolution bath sets new standards for precise, quick and easy operation. Like its predecessor models, the AT dissolution bath has been optimized for ideal water circulation to ensure extremely high temperature homogeneity during the test. From fast filling and draining of the water bath with quick-connector, self-priming circulation pump, to motorized lift function, all handling and cleaning processes of the AT have been optimized for simple and fast execution. When adding test medium or removing vessels, the vertically lifted shaft drive provides excellent accessibility during all change-over and cleaning processes.
Compliance by design
The AT dissolution apparatus fully complies with all harmonized Pharmacopeia requirements and features “built-in” compliance without any adjustment. The proven SOTAX AutoCompliance™ concept with fixed shaft height and vessel positioning guarantees 100% compliance without requiring time-consuming adjustments by the operator. For fast change-overs, quick-lock systems and a vertical bath closing mechanism (manual or motorized) provide for simple handling processes.
Auto-centering vessels: Time-consuming vessel set-up is unknown with the AT’s auto-centering quick-lock system. Locking rings, which are fixed on the base plate, guarantee highly accurate vessel centering and allow analysts to insert or remove vessels within seconds. Each vessel is certified and closed with an individual cover limiting evaporation.
Fixed shaft height: Proven to have the lowest wobble ratings in the industry, the AT incorporates the same shaft design as previous SOTAX dissolution baths. All shafts are self-centering and no adjustment of shaft height is required – maintaining the highest level of precision while making changeovers fast and simple.
Fully documented qualification:
The AT has been designed to facilitate highly accurate mechanical calibration anytime. Using the innovative Mechanical Qualification Device (MQD), all required procedures and measurements can be easily performed and results are automatically documented according to the current FDA guidelines on enhanced mechanical qualification.
Flexibility maximized - suited for all dosage forms
Large inlet dimensions provide for a wide variety of tablet and sinker types to be introduced manually or automatically. From standard tablets to capsules with large sinkers – the dissolution bath is capable of handling virtually all dosage forms. Samples are protected from media before test start. As the inlets are closed during the test, media evaporation is prevented.
Flexibility maximized - various vessel styles
For full flexibility, the dissolution apparatus can be operated with various vessel types. The bath accommodates a broad variety of different vessel styles – from certified standard glass vessels to polycarbonate or low actinic glass vessels, 1 or 2 liter vessels, mini vessels, immersion cell vessel, peak vessels, and China vessels.
Flexibility maximized - USP 1,2,5,6 methods and more
Developed for USP 1,2,5,6 methods, the dissolution apparatus allows testing with different compliant and also non-compliant stirrer designs: Basket, Paddle, Paddle over disk, Rotating Cylinder, Extraction Cell, Mini Paddle, China Paddle, Stationary Basket, Intrinsic Dissolution. All parts are made of high quality material, are serialized and certified.
Complete Sampling Solutions
Automating the sampling process improves accuracy, reproducibility and simplifies method transfer. Standardized modules for sample withdrawal allow sampling tailored to your application. For dissolution sample withdrawal, four key factors must be considered: height reproducibility, automation potential, hydrodynamic impact and possible coupling to temperature monitoring. Automating the process with simultaneous sampling and automatic pumping improves time and volume accuracy. Based on your requirements, SOTAX dissolution baths offer a wide variety of manual and automatic sampling possibilities:
Simple manual sample withdrawal using a glass pipette
Stand-alone type sampling probe with additional pipette guide
Stationary cannula in different materials and diameters for sampling with disposable syringes
Automatic simultaneous sampling with the unique HollowShaft™ – reducing hydrodynamic perturbation
Automatic simultaneous sampling with cannula and AutoLift™ (AT Xtend™ only) for maximum flexibility
UV-Vis analysis on the fly
The dissolution software used in the UV-Vis on-line systems gives the lab scientist dissolution results on the fly. From a mono- or multi-wavelength absorbance measurement - compared to a standard value stored or measured - concentration and % dissolved are immediately calculated, stored and reported.
Off-line analysis
Workload increase or method changes often call for maximum flexibility and modular and scalable sample management devices. They also require safe and reproducible collection and storing as well as logical programming. An off-line configuration offers simplicity and flexibility as the samples once stored in glass tubes or LC vials can be conveyed to any kind of analytical device in all kind laboratory set-ups.
Components
AT Dissolution Bath

Built on the success of preceding SOTAX dissolution systems, the AT can be flexibly configured for USP 1,2,5,6 dissolution methods. Its unique design combines robust quality components with state-of-the-art technology to guarantee reproducible testing conditions, day after day. Depending on the required automation level, the AT can be flexibly extended with additional Xtend™ modules – making method transfer for increased throughput requirements easier than ever.
CP Piston Pump Module

Simultaneous Sample Withdrawal
The accuracy of sample transfer from the dissolution apparatus to the sample manager and/or analytical device largely depends on the accuracy of the pump used and on its ability to push or pull liquids through the filters.
As sample transfer occurs simultaneously on all channels, any pump used must contain 6-8 equivalent modules.
The CP Piston Pump Module is the ideal choice for automated systems as it can be combined with the FS Filter Station, SAM Sample Manager or C61x Fraction Collector, and a UV-Vis spectrophotometer. It can also be used to perform media addition. To reduce the overall footprint, the CP Module is fully stackable on the other Xtend™ Modules. The ceramic piston head design is completely maintenance-free and does not require any priming. Fully controlled by either the EasyTouch™ user interface or WinSOTAXplus Dissolution Software, the powerful CP Module can pull and push through filters with a porosity down to 0.2 microns.
UV-Vis Sample Analysis and Reporting

UV-Vis analysis being the final step of the dissolution process, SOTAX dissolution systems allow integration of different analytical processes and combine results in a powerful data management software.
The WinSOTAXplus Dissolution Software allows full control of all common UV-Vis spectrophotometers. Dissolution samples can be withdrawn, filtered, conveyed, and measured in “Absorbance” at single- or multiwavelength in flow through cuvettes. The readings are calculated, reported, and stored in a 21 CFR, Part 11 compliant SQL database.
Seamless integration with Specord Plus®
SOTAX also offers its own UV-Vis solution: the Specord Plus 200 or Specord Plus 210 UV-Vis spectrophotometers are fully integrated in the dissolution systems. Both devices exceed Pharmacopeia requirements and have a true double beam with reference compensation. Using two symmetrical 8-position cell changers, they are ideal for double on-line and double on-/off-line system
configurations. As a standard, the Specord Plus series comes with a 10 year warranty on optics.
SAM Sample Manager (Off-line/On-line)

Universal and Efficient Sample Management
Workload increase or method changes often call for maximum flexibility and scalable sample management – in addition to safe and reproducible collection & storage.
The SAM Sample Manager can be used either as a simple fraction collector to collect and store samples in standardized vials and tubes, or as an advanced sample manager to add or replace media. Controlled by the EasyTouch™ user interface or by WinSOTAXplus Dissolution Software, the SAM will fit with all dissolution methods – independent of the automation level chosen.
The SAM module can be stacked with the CP Piston Pump module and/or the FS Filter Station to reduce overall system footprint.
Available in three versions (S/M/L), the module allows vertical storage of samples in up to 3 levels. Its capacity can also be extended horizontally for double-automated systems or for an increased number of time points.
WinSOTAXplus Advanced Dissolution Software
Professional Data Management
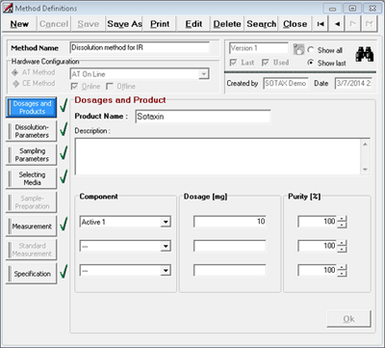
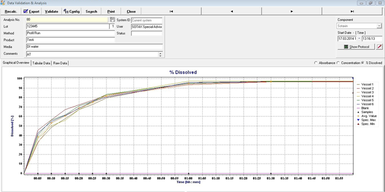
Developed in close cooperation with industry experts and system users, theWinSOTAXplus software is based on a deep understanding of today's dissolution user requirements and allows implementation of a fully 21 CFR, Part 11 compliant data management system for all XtendTM configurations and automation levels.
The WinSOTAXplus software offers user-friendly control of your complete system. Whether used stand-alone or in a networked environment, the modern client-server architecture can be flexibly implemented for different needs. From data capture to analysis with customized reporting and exporting features - the software fulfills all R&D and QC requirements.
For advanced data management, specific user-group definition and bi-directional data transfer to ELN/LIMS are available. Simple batch analysis alongside assisted data review is possible with customized reports and method creation including product specifications. Spend less time on compliance paperwork with highlighted pass/fail reports alongside enhanced temperature, rpm, and pH recording throughout the test. Comprehensive standard monitoring and customized system suitability requirements are stored and executed per product to ensure proper SOP execution.
Optional Components for Your Configuration
MPS Media Preparation Station

Accurate media preparation plays a vital role in all subsequent processes. The MPS Media Preparation Station allows operators to degas, heat, and dispense the exact volume of dissolution media required for each method.
Degassing at the USP and FDA recommended levels, the MPS is a mobile unit assisting in bringing 10 – 20 L of degassed and heated media to your dissolution system. By inserting the dispensing nozzle into individual vessels, precise and gravimetrically validated dispensing of media at 1’500 mL per minute without re-aeration is achieved. Once dispensing has been completed, the MPS can be simply connected to the next DI water inlet to start its automated cleaning routine – while the dissolution test runs.
FS Filter Station

Filtering dissolution samples prior to analysis is essential to the accuracy of dissolution results. The Xtend™ Dissolution Line can be operated with different membranes and filters of various porosities.
Membrane filters: The 25 mm membrane filters can be used with sampling probes, automated cannulas, and HollowShaft™. Integration directly into the suction head makes these filters both economical and user friendly.
Syringe Filters: The 25 mm syringe filters offer 100% flexibility as they can be universally used on disposable syringes, sampling probes, stationary cannulas, automated cannulas, HollowShaft™, CP Modules, and FS Filter Stations. Ideal for simplified method transfer, they can be used on all automation levels. During method development in R&D, the variety of porosities allow the user to find the filter required for analytical finish. In QC, the robustness and strength of this filter housing guarantees consistency of the filtration surface. Syringe filters are Luer-locked and therefore robust and easy to use.
FS Filter Station: To avoid saturation and clogging of syringe filters, it may be necessary to exchange them after each sampling point. The FS Filter Station automatically exchanges filters and can hold up to 424 filters for filtering through 6, 7, or 8 channels. Robust and fast in operation, the module guarantees liquid-tight circulation at each time point. To reduce overall system footprint, it can be stacked with other Xtend™ Modules.
Xtend™ Dissolution Line
English Brochure
Тестер "Растворение"
Брошюра на русском языке на серию тестеров Расторения XTEND
To get information about: «Semi-automated (Double System)» contact us by phone (044) 229 15 31 or fill in the form.